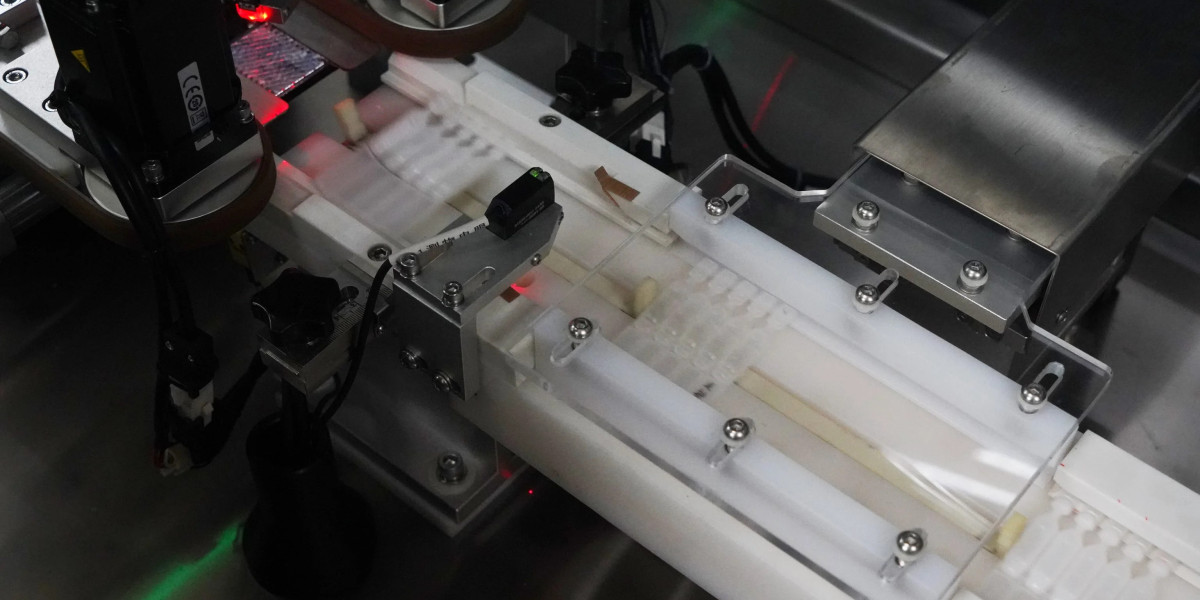
In the ophthalmic product market, sterility and seal integrity are non-negotiable. Single-use eyedrop containers, typically produced by the BFS (Blow-Fill-Seal) process, are designed to eliminate the need for preservatives and reduce contamination risk. However, even microscopic leaks can compromise these benefits—posing significant risks to product safety and patient health.
The Single-Use Eyedrops Leak Detection Machine provides an advanced, non-destructive solution to identify such leaks with exceptional precision, ensuring that every unit leaving the factory meets the highest quality standards.
The Problem: Why Traditional Methods Fall Short
Conventional inspection techniques like dye penetration are outdated for high-speed BFS production. They are slow, prone to contamination, and incapable of detecting micro-defects below 10 μm. Moreover, destructive methods mean tested samples must be discarded—wasting both product and packaging.
For enterprises producing millions of units daily, these inefficiencies translate directly into higher costs and compliance risks.
How High-Voltage Non-Destructive Testing Works
This equipment applies high-voltage discharge sealability detection to inspect each eyedrop container individually:
A controlled high-voltage electric field is applied between two electrodes.
If the container wall is intact, no current flows.
When a hole or crack exists, current passes through the defect and is detected instantly.
The system differentiates between normal and defective containers based on current variations.
This method enables micro-leak detection down to 5.0 μm, without physical contact or chemical contamination.
Key Technical Advantages
Non-Destructive Testing: Each product remains fully usable after inspection, avoiding waste.
Zero Contamination Risk: No dye or immersion liquid required.
High Sensitivity and Accuracy: Detects minute micro-leaks invisible to human eyes.
Safe for Plastic Ampoules: Low discharge energy ensures no heat or structural damage to BFS containers.
Continuous Inline Inspection: Suitable for integration into automated packaging lines.
Industrial Applications
This system is particularly valuable for:
Pharmaceutical manufacturers producing ophthalmic preparations in BFS packaging.
Contract manufacturers (CMOs) handling multiple small-volume liquid products.
Quality assurance laboratories that need non-destructive verification tools for packaging validation.
Benefits for Enterprises
Adopting this leak detection system enables companies to:
Achieve consistent product quality across batches.
Reduce manual inspection labour and improve throughput.
Comply with international GMP and regulatory standards.
Protect brand credibility through proven packaging reliability.
Conclusion
In an industry where patient safety and regulatory compliance drive every decision, the Single-Use Eyedrops Leak Detection Machine delivers a reliable, data-driven approach to packaging integrity testing.
By leveraging non-destructive high-voltage technology, pharmaceutical enterprises can move beyond traditional leak tests—achieving precision, safety, and efficiency in one system.